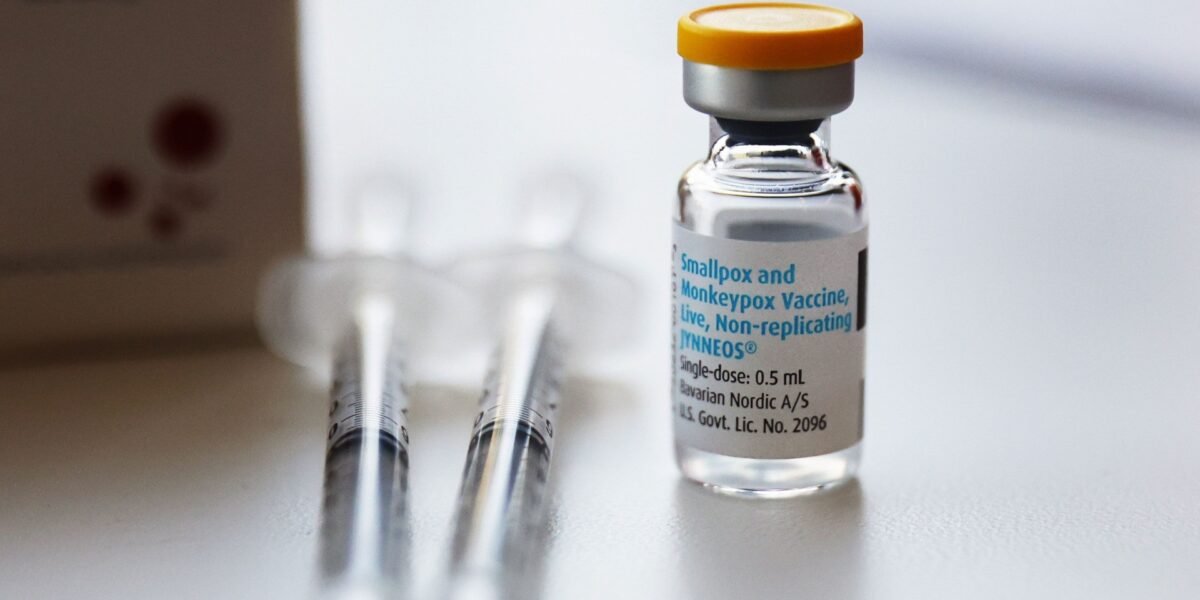
The World Health Organisation (WHO) has announced the MVA-BN vaccine as the first vaccine against monkeypox to be added to its prequalification list, says the organisation.
The prequalification approval is expected to facilitate timely and increased access to this vital product in communities with urgent need, to reduce transmission and help contain the outbreak.
WHO Director-General Dr Tedros Adhanom Ghebreyesus said, “This first prequalification of a vaccine against mpox is an important step in our fight against the disease, both in the context of the current outbreaks in Africa, and in future.”
Read more: Fifth Mpox case confirmed in KP, says health minister
The MVA-BN vaccine can be administered in people over 18-years of age as a 2-dose injection given 4 weeks apart.
According to WHO, a single-dose MVA-BN vaccine given before exposure has an estimated 76 per cent effectiveness in protecting people against mpox, with the 2-dose schedule achieving an estimated 82 per cent effectiveness. Vaccination after exposure is less effective than pre-exposure vaccination.
Earlier, the World Health Organisation (WHO) declared the recent outbreak of the disease a public health emergency of international concern after a new variant was identified.